The once-daily dose of CYCLOSET®, taken within 2 hours of waking, reduced PPG at 1 and 2 hours after each meal. CYCLOSET® helped reduce fasting and postprandial hyperglycemia throughout the meals of the day—without raising plasma insulin levels.1
DISCOVER THE EFFICACY OF CYCLOSET®1
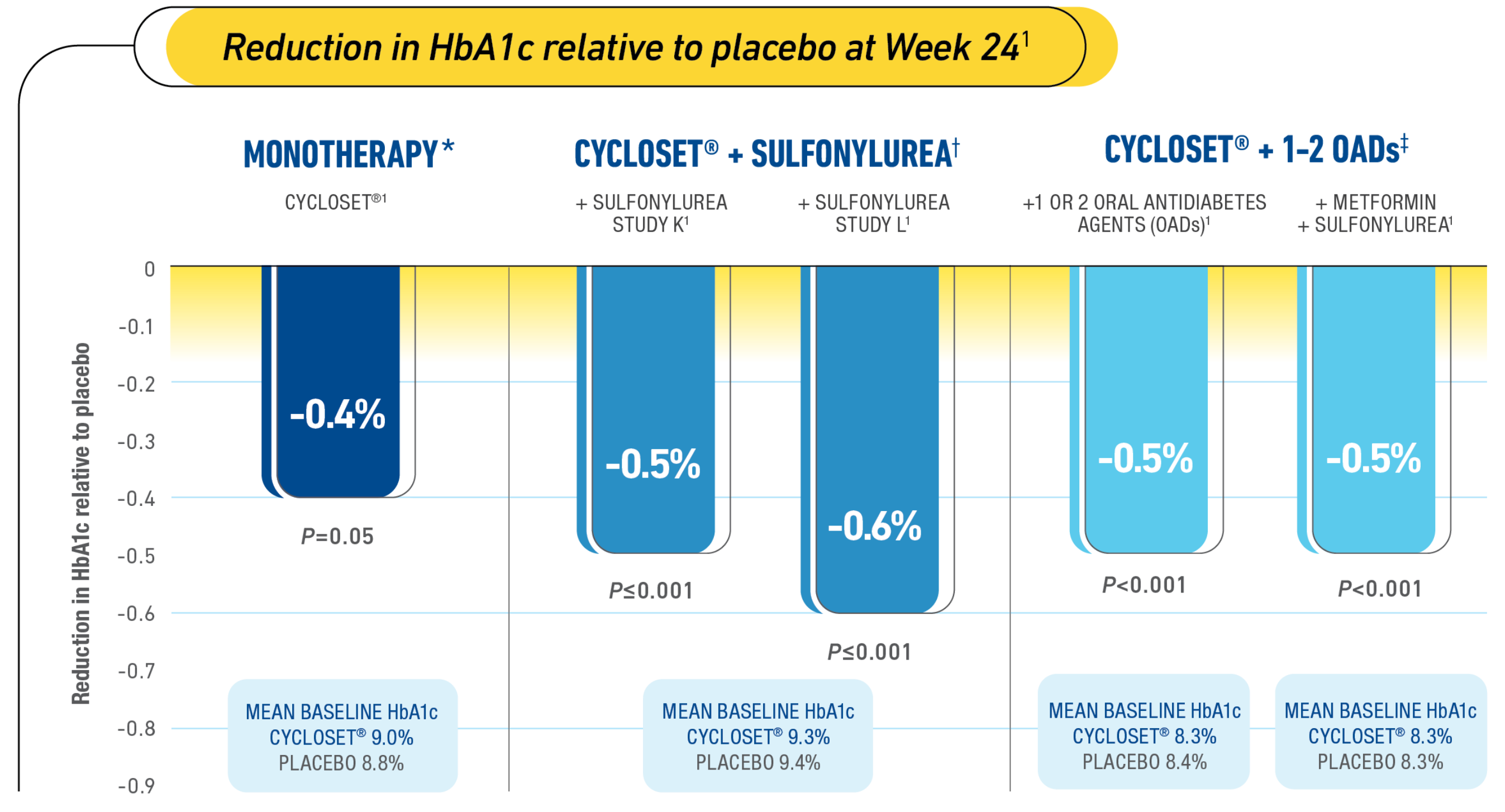
Proven HbA1c reduction when added glycemic control is needed1
Complementary efficacy of CYCLOSET® in clinical studies1
HbA1c=hemoglobin A1C.
*Study Design (CYCLOSET® Monotherapy): A 24-week, multicenter, double-blind, placebo-controlled study. CYCLOSET® monotherapy, n=80; placebo, n=79. At baseline and Week 24, patients received standardized meals at 7 AM (breakfast), 12 PM (lunch), and 5 PM (dinner). Blood samples were taken prior to and 1 and 2 hours following each meal and analyzed for insulin and glucose concentrations. Premeal plasma glucose levels at Week 24: fasting: CYCLOSET®=-2 mg/dL, placebo=+28 mg/dL; lunch: CYCLOSET®=-16 mg/dL, placebo=+15 mg/dL; dinner: CYCLOSET®=-2 mg/dL, placebo=+13 mg/dL.1-3
†Study Design (CYCLOSET® + Sulfonylurea): Two 24-week, multicenter, placebo-controlled, double-blind studies. The primary endpoint was reduction in HbA1c relative to placebo. Study K: CYCLOSET®, n=114; placebo, n=122. Study L: CYCLOSET®, n=114; placebo, n=123. Intent-to-treat population using last observation carried forward between-group change from baseline in HbA1c.1,3
‡Study Design (CYCLOSET® + 1-2 OADs): A 52-week, randomized, double-blind, multicenter, placebo-controlled safety study with subgroup efficacy assessments at Week 24. CYCLOSET® or placebo + 1 or 2 OAD medications, n=376; placebo, n=183. CYCLOSET® + metformin + sulfonylurea, n=177; placebo, n=90. CYCLOSET® + thiazolidinedione (TZD) +/- OAD, n=78; placebo, n=44. Patients in the “metformin + sulfonylurea” and “TZD +/- OAD” subgroups were also counted in the “adjunct to 1 to 2 OAD” subgroup. OADs included metformin, sulfonylurea, alpha-glucosidase inhibitor, meglitinide, phenylalanine derivative, or oral combination therapy formulated as 1 pill. Doses of background antidiabetic medications could be adjusted at any time during the trial, and additional antidiabetic medications were permitted after Week 12, if needed to maintain ideal glycemic control.1,2,4-6
In a pharmacodynamic study:
The CNS-acting insulin sensitizer helped reduce postprandial plasma glucose (PPG)1
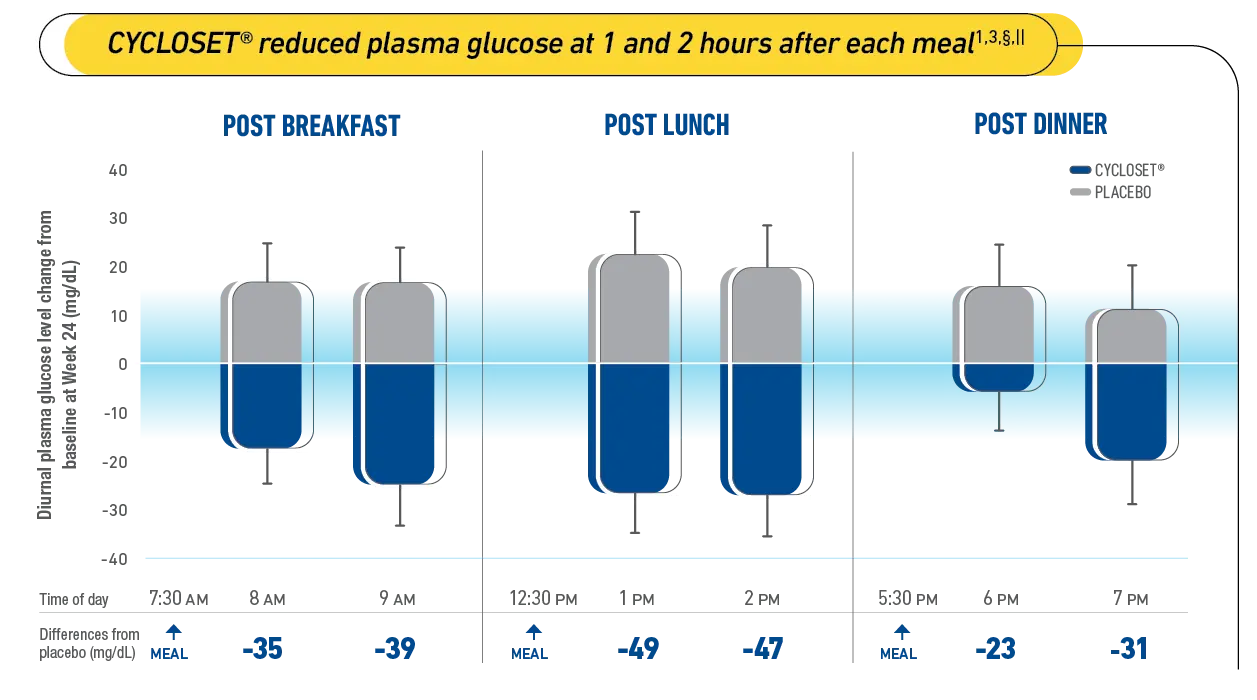
§Study Design: Patients with type 2 diabetes mellitus (T2DM) on diet therapy alone and with poor glycemic control were randomized to treatment with CYCLOSET® or placebo (baseline fasting plasma glucose: 214 ± 6 and 203 ± 6 mg/dL; HbA1c: 9.0 ± 0.1 and 8.8 ± 0.1, respectively) and administered standardized meals at breakfast, lunch, and dinner, before and 24 weeks following treatment. Plasma samples taken before and 1 and 2 hours after each meal were analyzed for glucose and insulin.3
||A 2-way repeated-measures (ANOVA) analysis of treatment and hour indicate a significant treatment effect over the entire diurnal (7 AM to 7 PM) period (P=0.0012). The change from baseline in 2-hour glucose level is significantly different for CYCLOSET® vs placebo at all 3 meals (P<0.05). The improvements on diurnal and postprandial glucose were not associated with an increase in insulin level measured at any of these test times.3
INDICATION
CYCLOSET® (bromocriptine mesylate) 0.8 mg tablets is a dopamine receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
LIMITATIONS OF USE
- CYCLOSET should not be used to treat type 1 diabetes or diabetic ketoacidosis.
- Limited efficacy data in combination with thiazolidinediones.
- Efficacy has not been confirmed in combination with insulin.
IMPORTANT SAFETY INFORMATION
Contraindications
CYCLOSET is contraindicated in:
- Patients with hypersensitivity to ergot-related drugs, bromocriptine or to any of the excipients in CYCLOSET.
- Patients with syncopal migraines. May precipitate hypotension.
- Postpartum patients. Serious and life-threatening adverse reactions have been reported.
- Lactating patients. CYCLOSET contains bromocriptine which inhibits lactation.
References: 1. CYCLOSET [prescribing information]. Tiverton, RI: VeroScience LLC; 2020. 2. Cincotta AH, Meier AH, Cincotta M Jr. Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs. 1999;8(10):1683-1707. 3. Data on file. Salix Pharmaceuticals. 4. Florez H, Scranton R, Farwell WR, et al. Randomized clinical trial assessing the efficacy and safety of bromocriptine-QR when added to ongoing thiazolidinedione therapy in patients with type 2 diabetes mellitus. J Diabetes Metab. 2011;2(7):1-8. 5. Vinik AI, Cincotta AH, Scranton RE, et al. Effect of bromocriptine-QR on glycemic control in subjects with uncontrolled hyperglycemia on one or two oral anti-diabetes agents. Endocr Pract. 2012;18(6):931-943. 6. Scranton RE, Gaziano JM, Rutty D, Ezrokhi M, Cincotta A. A randomized, double-blind, placebo-controlled trial to assess safety and tolerability during treatment of type 2 diabetes with usual diabetes therapy and either Cycloset or placebo. BMC Endocr Disord. 2007;7(1):3.



