While CYCLOSET® is not indicated to reduce the risk of macrovascular events (coronary, cerebrovascular, and peripheral vascular), this study demonstrated its CV safety.1,2
UNDERSTANDING CYCLOSET® SAFETY AND OTHER FINDINGS1-3
The CYCLOSET® Trifecta1-3
In clinical studies, CYCLOSET® had additional safety and other findings on a trio of pathophysiologic parameters of T2DM.1-3


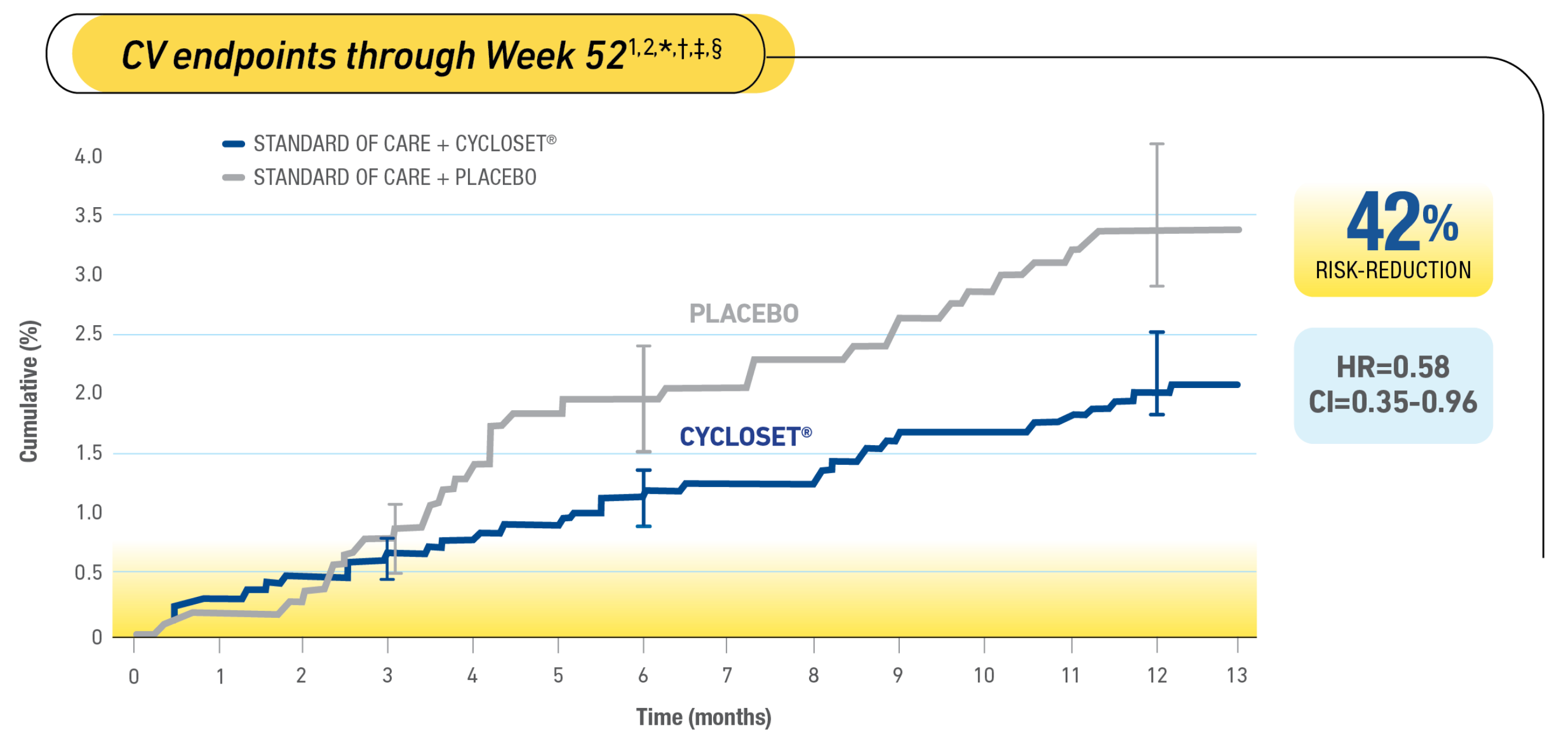
In a safety trial, CYCLOSET® demonstrated a 42% reduced CV risk vs placebo within 1 year1,2
CI=confidence interval; HR=hazard ratio; T2DM=type 2 diabetes mellitus.
*Study Design: Double-blind, 52-week (12-month), placebo-controlled, 2:1 CYCLOSET®-to-placebo randomized, outpatient, safety and noninferiority study in 3070 patients with T2DM. CYCLOSET®, n=2054; placebo, n=1016. One-third of patients had preexisting cardiovascular disease (CVD); 75% of patients had preexisting hypertension.1,3
†Prespecified independently adjudicated composite CVD endpoint: first myocardial infarction, stroke, coronary revascularization, or hospitalization for angina or congestive heart failure.1 Frequency of serious adverse events and time to first composite CV event were coprimary endpoints.1
‡The primary purpose of the study was to establish the safety profile of CYCLOSET®.3
§In a prospective, randomized, 1-year trial of 3070 patients with T2DM, CYCLOSET® use was associated with a hazard ratio of 0.58 (2-sided 95% CI, 0.35-0.96) for the time-to-first-occurrence of the prespecified composite CV endpoint of MI, stroke, coronary revascularization, or hospitalization for angina or congestive heart failure.1,3


CYCLOSET® showed a reduction in triglycerides2
Change in lipid levels relative to placebo2,||,¶
||Study Design: A 24-week, multicenter, placebo-controlled, double-blind study. The primary endpoint was reduction in HbA1c relative to placebo. Study L: CYCLOSET®, n=114; placebo, n=123. Intent-to-treat population using last observation carried forward between-group change from baseline in HbA1c.1,2
¶Mean baseline triglyceride levels of 250 mg/dL and free fatty acid levels of 800 μEq/L.4
#Secondary efficacy endpoint of the two 24-week efficacy studies as adjunct to stable sulfonylurea.2,4

Impact of CYCLOSET® on blood pressure3
CYCLOSET® has not demonstrated an unfavorable hypertensive effect on blood pressure.3 However, hypotension has been reported with use of CYCLOSET® in clinical trials.1
Safety analysis: CYCLOSET® blood pressure observations vs placebo3,**
mm Hg=millimeters of mercury.
**Study Design: Double-blind, 52-week, placebo-controlled, 2:1 CYCLOSET®-to-placebo randomized, outpatient, noninferiority, safety study in 3070 patients with T2DM. CYCLOSET®, n=2054; placebo, n=1016. Primary outcome measures were incidence of serious adverse events and a composite endpoint of CV events. Baseline CYCLOSET® systolic blood pressure (SBP) and diastolic blood pressure (DBP) were 130/78. Baseline placebo SBP/DBP were 130/77.3


- Hypotension, including orthostatic hypotension, can occur, particularly upon initiation of CYCLOSET® and with dose escalation. Use caution in patients taking antihypertensive medications1
- Although CYCLOSET® is not indicated to reduce blood pressure or plasma lipids, patients saw reductions in clinical trials1,3
Discover the well-established safety profile of CYCLOSET®1
Safety trial results for key T2DM treatment markers1,††,‡‡
CYCLOSET® was comparable to placebo in incidence of hypoglycemia1:
- 6.9% (142/2054) of CYCLOSET® patients vs 5.3% (54/1016) of placebo patients
CYCLOSET® and placebo showed similar mean changes in body weight1:
- +0.2 kg for CYCLOSET® patients vs +0.1 kg for placebo patients
††No pharmacokinetic studies have been conducted in patients with renal impairment. Although the kidney is a minor pathway for elimination of CYCLOSET®, caution should be used in patients with renal impairment.1
‡‡Efficacy has not been confirmed in combination with insulin.1
In the 52-week CYCLOSET® safety trial, the primary endpoint of the occurrence of all-cause SAEs was not different between CYCLOSET® and placebo groups1
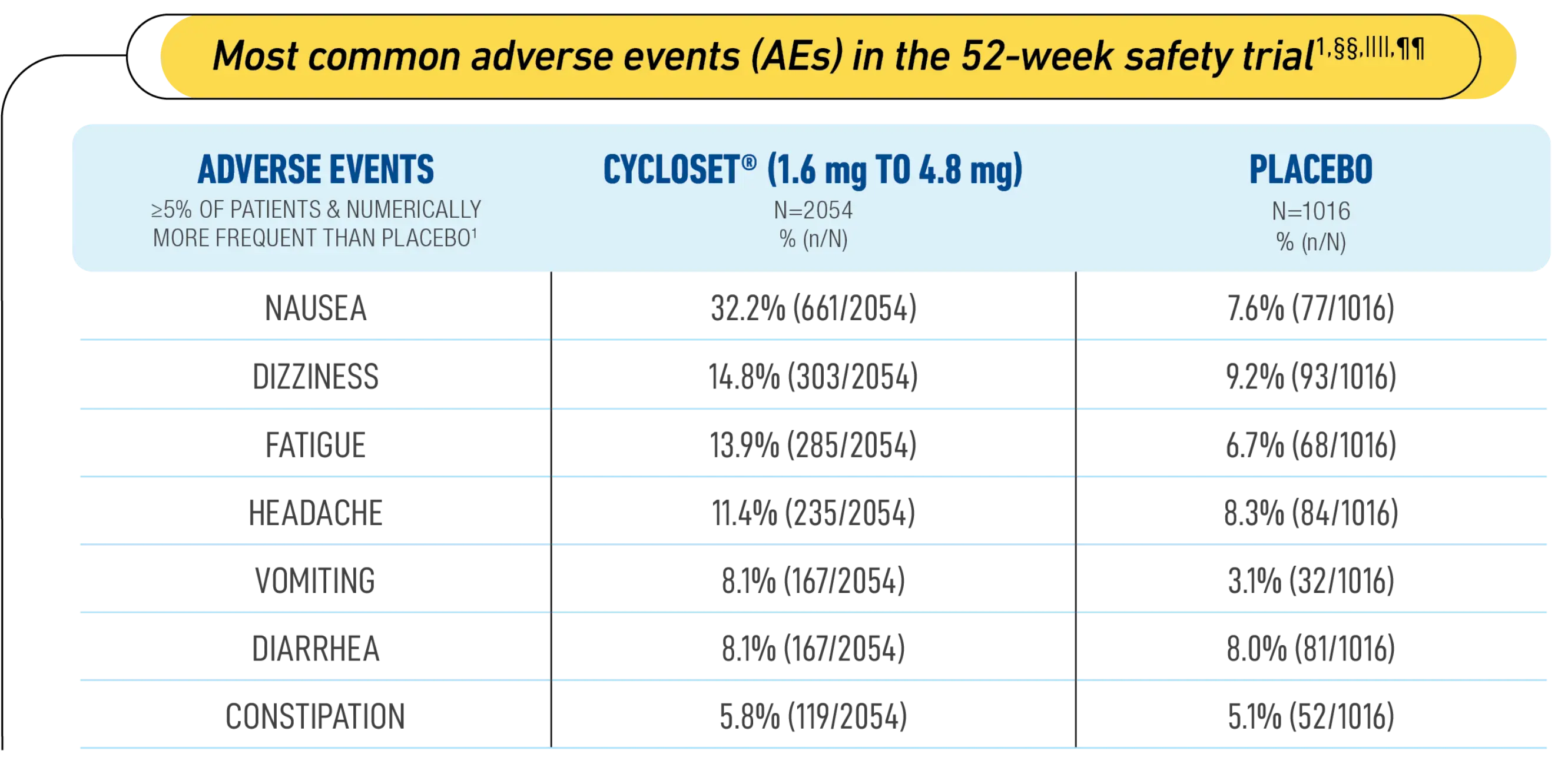
These AEs usually occurred during initial titration and lasted a median of 14 days1
SAE=serious adverse event; T2DM=type 2 diabetes mellitus.
§§Study Design: A 52-week, randomized, double-blind, placebo-controlled safety study of 3070 patients. Patients were treated with diet or no more than 2 antidiabetic medications (metformin, insulin secretagogues such as sulfonylurea, thiazolidinediones [TZDs], alpha-glucosidase inhibitors, and/or insulin). The primary endpoint of the 52-week CYCLOSET® safety trial was the occurrence of all-cause serious adverse events (SAEs). SAEs occurred in 8.5% (176/2054) of CYCLOSET® patients vs 9.6% (98/1016) of placebo-treated patients.1,5
||||Post-marketing reports with higher doses of bromocriptine used for other indications include psychotic disorders, hallucinations, and fibrotic complications.1
¶¶Data from a 52-week safety study. All randomized subjects (N=3070) received at least 1 dose of study drug. The safety trial enrolled patients (CYCLOSET®, n=2054; placebo, n=1016) treated with diet or no more than 2 antidiabetic medications (metformin, insulin secretagogues such as sulfonylurea, TZDs, alpha-glucosidase inhibitors, and/or insulin). Primary endpoint of the 52-week CYCLOSET® Safety Trial was the occurrence of all SAEs.1
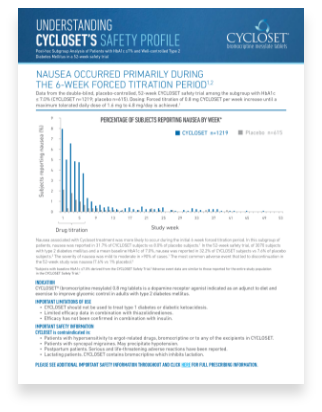
Learn more about nausea in the CYCLOSET® 52-week safety trial with this downloadable card.
INDICATION
CYCLOSET® (bromocriptine mesylate) 0.8 mg tablets is a dopamine receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
LIMITATIONS OF USE
- CYCLOSET should not be used to treat type 1 diabetes or diabetic ketoacidosis.
- Limited efficacy data in combination with thiazolidinediones.
- Efficacy has not been confirmed in combination with insulin.
IMPORTANT SAFETY INFORMATION
Contraindications
CYCLOSET is contraindicated in:
- Patients with hypersensitivity to ergot-related drugs, bromocriptine or to any of the excipients in CYCLOSET.
- Patients with syncopal migraines. May precipitate hypotension.
- Postpartum patients. Serious and life-threatening adverse reactions have been reported.
- Lactating patients. CYCLOSET contains bromocriptine which inhibits lactation.
References: 1. CYCLOSET [prescribing information]. Tiverton, RI: VeroScience LLC; 2020. 2. Data on file. Salix Pharmaceuticals. 3. Gaziano JM, Cincotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care. 2010;33(7):1503-1508. 4. Cincotta AH, Meier AH, Cincotta M Jr. Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs. 1999;8(10):1683-1707. 5. Scranton RE, Gaziano JM, Rutty D, Ezrokhi M, Cincotta A. A randomized, double-blind, placebo-controlled trial to assess safety and tolerability during treatment of type 2 diabetes with usual diabetes therapy and either Cycloset or placebo. BMC Endocr Disord. 2007;7(1):3.